MedChem Tips and Tricks
Produced by the ACS GCI Pharmaceutical Roundtable subteam on Medicinal Chemistry
Last updated: February 12, 2016
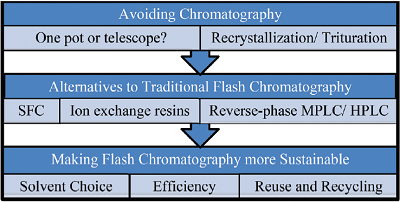
“Green Chromatography” Decision Tree
See: Green Chem. (2014) 16, 4060
Extractions/work-up conditions
- If your material is not sensitive to base, potassium carbonate can be a better ‘salting out’ additive than the more usual sodium chloride. Potassium carbonate has very high solubility in water and of course higher ‘ionic strength’ to ‘push’ your organic material out of the water.
- Aqueous acetic acid wash can work better than HCl (aq) for purging low MW amines, such as Hünig’s base.
- Dipolar Aprotic Solvent (DAS) extractions: can often be problematic (either extracting product effectively, or carrying through of undesired DAS solvent). Process typically involves quench into water, followed by filtration or extraction to remove product. Org. Process Res. Dev., 2007, 11 (1), pp 160–164
- Extract product with Least Polar solvents first (this will minimise levels of DMF / DMSO that follow your product)
- Heptanes or TBME works well (DMF is immiscible in these), Note: DCM gives poor results.
- DMF can be azeotropically removed using n-heptane, xylene and ethylbenzene.
- DMSO/Hünig’s Base (DIPEA) separate into two-phase mixture. This can be used for extracting acids from DMSO mixtures, hence avoiding large (aq) volumes.
Bonded/ion exchange resins
- Can be used as loose resins for scavenging or on purification machines standard to the industry
- There are now a number of bonded columns available to help you separate compounds that pose a challenge on simple silica gel. Added bonus is that these columns can be reused up to 20 times!
- Review of ion exchange resins: Chem. Rev., 2009, 109 (2), 515-529
- SFC is not only for chiral separations
- Using alcohol solvents and CO2 recycling SFC has significantly less environmental impact than HPLC
- SFC can replace chlorinated solvent use for compounds with low solubility
Recrystallizations
Water wet solvents often solubilize compounds much better than dry solvents & should be considered when solubility screening. Note: You’ll be using water wet solvents for extraction! If your compound is soluble in a solvent when wet but insoluble when the solvent is dry this can be used to aid isolation or crystallization. Simply do your extractive work-up and azeotropically dry to crystallize your product. MEK (Methyl Ethyl Ketone) is particularly good for this.
Reverse Phase Chromatography
Purification of a variety of polar compounds on scale can now be easily carried out using C18 ISCO columns.
- less solvent/glass waste and shorter run time compared to multiple injections on prep HPLCs
- reusable columns reduce the amount of solid waste compared to silica columns
- no need to use DCM to purify polar compounds
Silica Gel Chromatography
- Solubility: it is useful to note that solubility in mixed solvent systems is often non-linear – a compound may be much more soluble in a 50:50 mixture of solvents than it is in either of the individual solvents. Ref: http://www.scientificupdate.co.uk/index.php/blog/item/solvent-work-up-tips.html
- Try using solvents mixtures (consider compatibilities) and running at higher concentration rather than simply diluting out (this can significantly aid workup efficiency and often lead to purification through evaporative crystallization).
- Pre-packed Silica Column reuse protocol:
- Using TLC as a guide, flush column with polar eluent to flush remaining material from column. Pre-columns/loading columns can be used as a way to minimize baseline material remaining on the column.
- Re-equilibrate with desired gradient mixture or flush with air to remove solvent for later use.
- Air pockets may form that will alter performance due to inconsistent packing of the stationary phase.
- DCM replacement in purification: DCM has an intrinsically high carbon footprint, is a greenhouse gas, has to be (expensively) scrubbed on incineration and chlorinated solvents have acute and chronic effects on human health. Solvent systems that can be used depending on solvents available include:
- 3:1 EtOAc in EtOH:
Relative Eluting Strengths of Green Chemistry Chromatography Solvent Mixtures
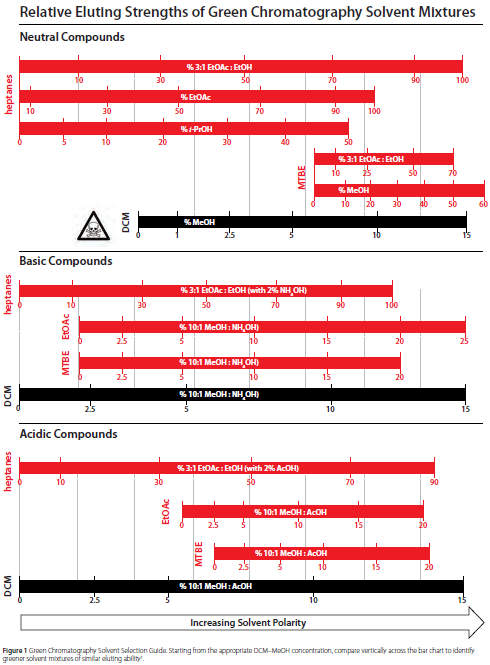
See: Green Chem., 2012, 14, 3020 and Sigma-Aldrich Greener Chromatography Solvents
2. 3:1 iPrOAc and MeOH in heptanes: “Dilution of MeOH with i-PrOAc enables better separation between closely spaced peaks”
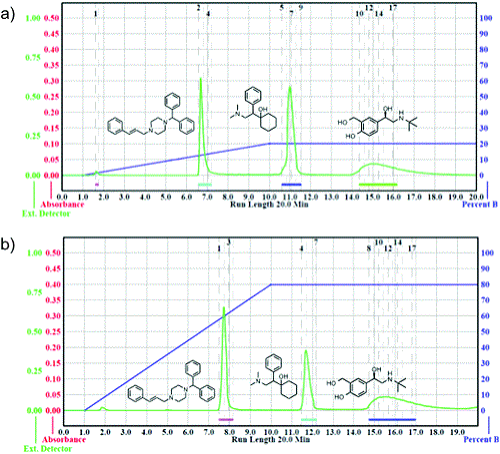
Image from Green Chem (2014) 16, 4102
Several excellent resources for solvent guides
n-Butanol
- Can be used effectively as a solvent for halogen displacements. Added bonus: product precipitated out of the reaction mixture.
Methyl tert-butyl ether
- Alternative to most ethers as it has a much reduced tendency to form peroxides
- Alternative to DCM in silica chromatography
- MTBE forms azeotropes with water (52.6 °C; 96.5% MTBE) and methanol (51.3 °C; 68.6% MTBE)
- Caution: MTBE unstable toward strong acids, reacts dangerously with bromine, potential endocrine disruptor
2-MeTHF
- Obtained from agricultural waste via a green process
- Forms bi-layer with water unlike THF so that reactions can be worked up without solvent switching.
- Many reagents available in 2-MeTHF
- Worked better for Me-ester formation in the presence of an aniline than DCE and prevented need for TMS-diazomethane
- Suitable replacement to THF or Dioxane and may be a viable replacement to DCMin a variety of reactions including:
- Amide/Sulfonamide formation
- Boc protection
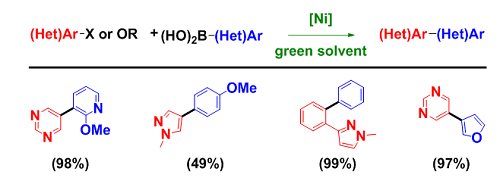
- Nickel-Catalyzed Suzuki–Miyaura Coupling: Org. Lett. 2013, 15, 3950
- Lactone formation using CDI : OPRD August 27, 2013
- Mitsunobu reaction, PPh3O crystallizes out and can be filtered off.
Heptanes
- Replacement for hexanes
- Better recrystallization solvent
- Can be used to azeotrope off DMF.
- Higher boiling point than EtOAc so that solvent removal from chromatography leaves solids instead of oils.
- Cyclohexanes also replacement for hexanes
Sulfolane
A highly stable alternative to DMSO, DMF, DMAC, and NMP
- The melting point of sulfolane can be lowered by addition of small amounts of water. Sulfolane with 3 vol % water has a melting point of 10 °C. This mixture is also commercially available. With 10 vol % of water the melting point is as low as 0 °C. Its viscosity is much higher than for the other dipolar aprotic solvents. However skin permeability of sulfolane is much lower than that of the other solvents.
- Due to the stability of sulfolane towards strong acidic conditions, even at high temperatures, it can be used with strong acids and also together with reagents such as phosphorous tri- and pentachloride or thionyl chloride. It has also been used as solvent for oxidations, nitrations, rearrangements, halogen exchange, phosphonylations, and condensation reactions
- Sulfolane is miscible with ester, ketone, and ether solvents in the same way as the other dipolar aprotic solvents. However sulfolane is not miscible with MTBE; in the case where the product acts as a detergent, addition of a small amount of water improves separation: Org. Proc. Res. Dev. 2012, 16 (7), pp 1273–1278
Dimethyl isosorbide
A sustainable solvent in skin-care products and drug formulation can be a suitable replacement for THF and dioxane in some reactions. Its high boiling point offers a wider range of reaction temperatures, but it is completely soluble in water.
IPAC
Is also a great solvent for triturations and extractions, retains less water than EtOAc and it is less reactive!
Lactic acid
As solvent: Green Chemistry Journal article on various annulations run in lactic acid, acting as a solvent and promoter, outperforming conventional organic solvents and acetic acid: Lactic acid as an invaluable bio-based solvent for organic reactions
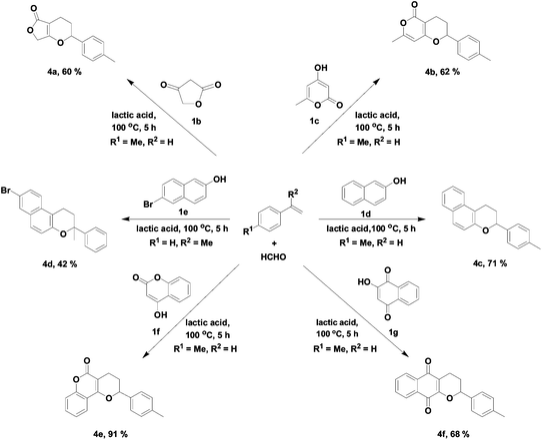
Reproduced by permission of The Royal Society of Chemistry
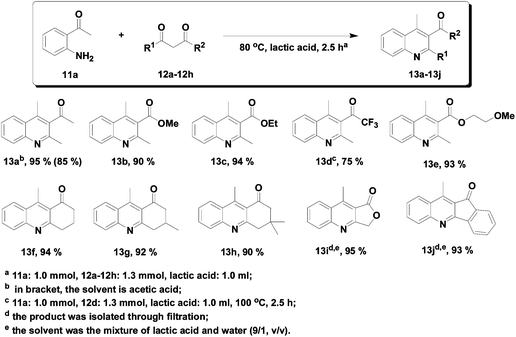
Reproduced by permission of The Royal Society of Chemistry
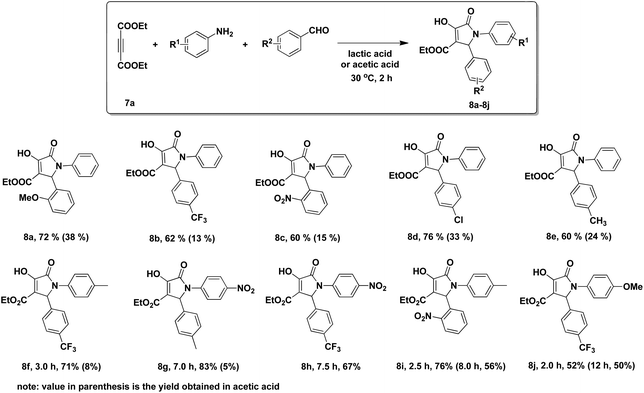
Reproduced by permission of The Royal Society of Chemistry
Greener solvents
For ruthenium and palladium-catalyzed aromatic C–H bond functionalization: Green Chem., 2011,13, 741-753, Green Chem., 2010,12, 2053-2063
Alternatives to DMF and DCM in Amide Coupling Reactions
- IPAC also works with HATU
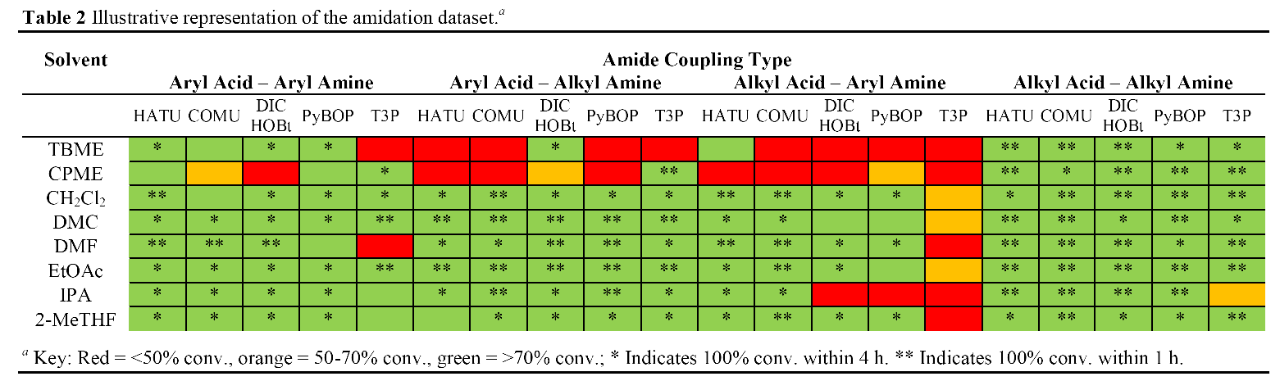
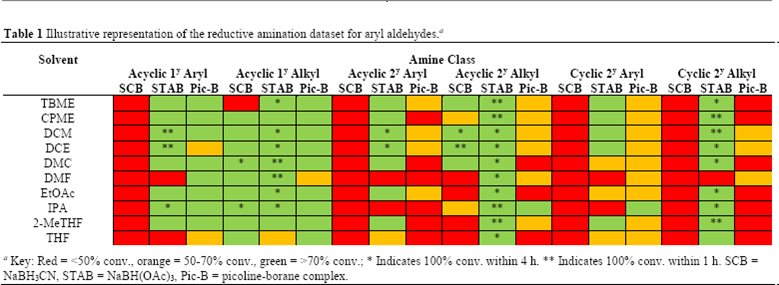
Reductive Amination
EtOAC and dimethyl carbonate are viable alternatives to chlorinated solvents: Development of a Solvent Selection Guide for Aldehyde-based Direct Reductive Amination Processes

Carbon tetrachloride
Can be replaced with dimethyl carbonate in a free radical bromination with NBS
TamiSolve® NxG
May be suitable replacement for NMP and NEP
- Taminco’s next-generation, high-performing, non-reprotoxic solvent
- Suitable NMP & NEP replacement (boiling point 241 C)
- Favorable safety, health and environmental profile: No reprotoxicity, biodegradable, no bio-accumulation, not aquatoxic, high flash point & low volatility
Propylphosphonic Acid Anhydride (T3P)
Useful for amide couplings, dehydrations (instead of POCl3) and heterocycle formation. Synlett, 2009, 3378-3382, Tetrahedron 65 (2009) 9989–9996.
Air-stable version of AlMe3 called DABAL-Me3
An adduct of trimethylaluminum and DABCO (http://www.sigmaaldrich.com/chemistry/chemical-synthesis/technology-spotlights/dabal.html) for Weinreb amidation of esters w/o the need of trimethyl aluminum, a highly flammable reagent
- Literature Reference for amide formation: Amide bond formation using an air-stable source of AlMe3:Method A: Reactions performed on a 0.50 mmol scale using amine (1.5 equiv), DABAL–Me3 1 (1.5 equiv), anhydrous THF, N2, 40 C, 1 h followed by ester (1 equiv), reflux, 18 h.
- Method B: Reactions performed on a 0.50 mmol scale using amine (1.5 equiv), DABAL–Me3 1 (1.5 equiv), undried THF, open to air, 40 C, 1 h followed by ester (1 equiv), reflux, 18 h.
Metal based oxidizing agent alternative
Stabilized IBX (SIBX, Aldrich-661384)
- SIBX can effectively oxidize alcohols to aldehydes and ketones, although sometimes heating is necessary.
- IBX alone is shock-sensitive and has been responsible for explosions, although they are believed to be due to residual potassium bromate left from its preparation. Neat material should not be heated above 30°C, however, mixtures in dilute (i.e. >5 volumes) in low boiling solvents (i.e. b.p < 100°C) can safety be heated to around 80°C.
- Compatible with variety of green solvents such as 2-MeTHF, toluene and EtOAcare compatible with SIBX use.
Enzyme Laccase (Aldrich)
Enzymatic oxidations to convert a benzylic alcohol into the corresponding aldehyde.
Zinc phthalocyanine (14320-04-8)(catalytic)
Reduced nitro groups in the presence of acid, amide, ester, halogens, lactone, nitrile, N-benzyl, O-benzyl and hydroxy groups
- To a mixture of nitro compound (1.34 mmol) and zinc phthalocyanine (1 mol%) in PEG-400 (3 ml) was added hydrazine hydrate (2 equiv.). The reaction mixture was stirred at 100 ºC for 8 h. Time was not optimized separately for all substrates. After completion of reaction, the reaction mixture was cooled to ambient temperature and 20 ml of ethyl acetate was added. PEG-400 was removed by washing with distilled water and ethyl acetate layer was dried under reduced pressure using rotatory evaporator and analyzed by GC-MS.
Stable and efficient triflation
4-Nitrophenyl trifluoromethanesulfonate and K2CO3 in IPAC upon heating produces triflation cleanly. The added bonus is the precipitation of the resulting potassium 4-nitrophenolate, which can then be filtered off along with K2CO3.
Suzuki−Miyaura Coupling over SiliaCat DPP-Pd
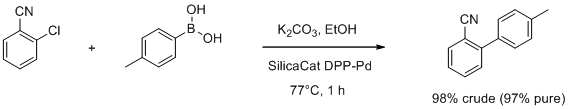
- The leach-proof and recyclable catalyst is available from Silicycle
Replacement of expensive and potentially explosive HOBt
With 2-hydroxypyridine in 2-MeTHF: J. Org. Chem. 2010, 75, 1155– 1161
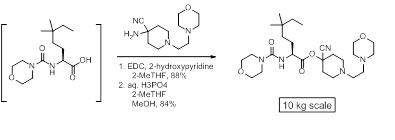
Highly efficient and environmentally benign
Preparation of Weinreb amides in the biphasic system 2-MeTHF/water: RSC Adv., 2013,3, 10158-10162
Preparation of Weinreb amides in the biphasic system 2-MeTHF/water
Trifluoromethylation of heterocycles in water at room temperature
Using a reaction medium containing nanoparticles consisting of commercially available TPGS-750-M in water, a combination of Langlois’ reagent and t-BuOOH can be used to affect trifluoromethylation of several heterocyclic arrays, including heteroaromatics. These reactions take place at ambient temperatures, and the aqueous medium can be recycled.
Dihydroxylations
(or oxidative (C=C) cleavage in combination with NaIO4) can be effectively catalyzed using an immobilized OsO4 (EnCat 40 – Reaxa/Aldrich) allowing safer application of this reagent. In an added bonus, the catalyst can be effectively recycled (via filtration and washing process) on multiple occasions with little / no leaching detectable or effect on performance. TIP: Use efficient mixing (preferably overhead stir) for best performance.
SMOPEX-301TM (J-M)
A triphenylphosphine functionalized fiber support (initially developed as a metal scavenger) can be used very effectively as a reagent replacement for triphenylphosphine reactions (Mitsonobu, Halogenation of alcohols, Wittig, tetrazole formation, etc) often resulting in a far simplified workup procedure (avoiding chromatography). The resultant phosphine oxide can simply be removed by filtration. It is available in bulk (key for scale up), and has good filtration properties being a fiber based resin. Ref:
Solubility of Inorganic component
(e.g. BASE or Metal Catalyst salt) in DAS solvent can often lead to reactions stalling. As reactions proceed, inorganic salt by-products form (e.g. NaCl). Only a certain ion concentration can be supported (solubilised) by the solvent medium, hence active component e.g. CO32-concentration can drop significantly during the course of a reaction (large effect on reaction rate!).
Process Tip: K2CO3 / Acetone reactions are well known for stalling on scale (or being slow). Consider adding:
- Phase Transfer Catalyst (Quaternary Ammonium Salts)
- Using Cs2CO3 – Increased organic solubility
- Using finer Mesh K2CO3 – increased surface area, combined with increased agitation rate.
Quenching Reactions
Water has a high surface tension, which makes it difficult to add in a controlled fashion. Controlled Addition of Water can be improved by using “wet organic mixtures” e.g. 10 % Water in THF. Very good for quenching very water-reactive reagents.
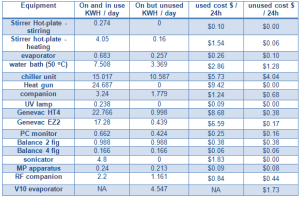
Energy usage by the numbers
- Leaving your Buchi chiller on all day uses the same amount of electricity as 4 loads in a tumble dryer.
- Leaving your Buchi water bath on all day at 50oC uses the same amount of electricity as doing 6 loads of washing
- One rotary evaporator left on (unused) consumes the same amount of energy as 10 TV’s left on
- Green Chemistry Wiki Page
- Green Chemistry Journal Home Page: http://pubs.rsc.org/en/journals/journalissues/gc#!recentarticles&all
- EROS – Encyclopedia of Reagents for Organic Synthesis: http://onlinelibrary.wiley.com/o/eros/eros_articles_fs.html
- Seven Important Elements for an Effective Green Chemistry Program: An IQ Consortium Perspective
- ACS GCI Pharma roundtable solvent selection guide
- E-Factor concept, and its effect on waste minimization in the Pharmaceutical Industry: The E Factor: fifteen years on: Green Chem. 2007, 9, 1273−1283.
- Chemical Watch article on Green chemistry: the role of EU regulation encompassing REACH