Peptides
Greener peptide manufacture is a fast-growing area of interest for the pharmaceutical industry and many companies have invested heavily in developing increased capabilities for peptide synthesis in-house and at contract manufacturing organizations. In recent years, there has been a growing interest in therapeutic peptides within the pharmaceutical industry with more than 100 peptide drugs approved for pharmaceutical use, and many more in clinical trials and preclinical development. The synthesis of peptides is associated with the use of comparatively high volumes of hazardous solvent and reagents and little focus on green chemistry and engineering.
In 2016, the ACS Green Chemistry Institute Pharmaceutical Roundtable identified development of greener processes for peptide API as a critical unmet need, and as a result, a new Roundtable focus team formed to address this important area. In a recent perspective (DOI: 10.1021/acs.joc.3c01494) the greener peptide team conducted a process mass intensity (PMI) assessment on 40 synthetic peptide processes at various development stages in pharma. This holistic assessment of the synthesis, purification, and isolation stages of peptide production demonstrated that solid phase peptide synthesis (SPPS), with a PMI ≈ 13,000, does not compare favorably with small molecule (PMI ≈ 168-308) or biopharmaceutical (PMI ≈ 8300) production. This assessment highlights the need for more environmentally friendly processes in peptide manufacturing.
This Roundtable team is organizing around benchmarking, sharing best practices, and identifying new synthetic methodologies in an attempt to reduce the environmental impacts associated with these processes.
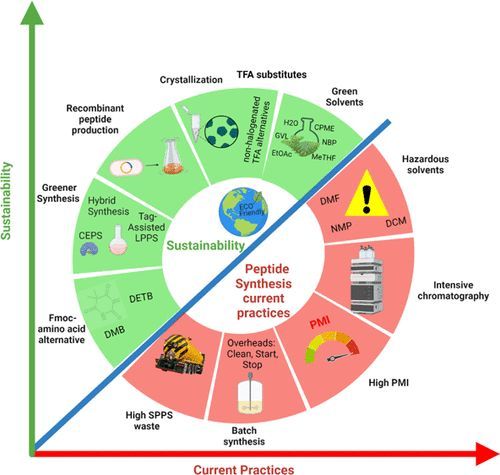
Image credit: DOI: 10.1021/acs.joc.3c01494